PCRs From PrimerStation960 Oligos
(For Printing On Arrayer)
- Assemble PCR cocktail without primers using Gibco BRL Platinum Taq (#10966-026 - also available in 250 U quantities on 4th floor Gibco Freezer, WAB 467B), for 100 ul PCR reaction:
- 10 ul PCR buffer, 10x
- 3 ul MgCl2
- 2 ul 10 mM dNTP (i.e., for ultimate concentration of 0.2 mM dNTP)
- 0.01 ul genomic DNA from MG1655
- 1 ul Taq
- 34 ul H2O
- 25 ul Primer 5'
- 25 ul Primer 3'
- Prepare PCR tubes on ice. Use Robbins Scientific 96 well plate that holds ~200 ul/well (for precipitation later on). Aliquot PCR cocktail, 50 ul each.
- Add 25 ul of both primers using multi-(12)-channel pipettor.
- Use PCR program:
- 95oC - 2 min
- 95oC - 30 sec
- 62oC - 30 sec
- 72oC - 2 min
- Go to step 2, 34x more
- 72oC - 2 min
- 4oC - forever
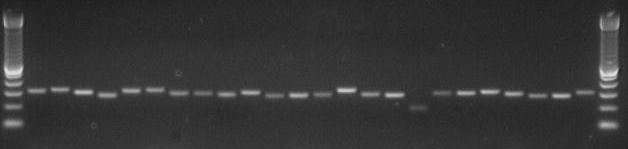
- Run out on 2% agarose: 1 ul from 100 ul PCR reaction. Use 100 bp ladder for JSE's 300-500 bp fragments.
PCR products from PS960 oligos generally look pretty good:
- Precipitate PCR products at -20oC for 1 hr:
- 10 ul 3 M sodium acetate
- 110 ul isopropanol (be sure to mix well)
- Spin at 3500 RPM in benchtop centrifuge for 1 hr, 4oC. Decant by flicking twice into sink. Pat dry on paper towels (ask Martin Steffen to demo wrist technique).
- Wash in 70% EtOH. Do NOT dilute down from 100% EtOH which contains benzene. Dilute down from 95%.
- Spin for 30 min at 3500 RPM, 4oC. Decant/flick into sink.
- Put into Speed Vac with Heater ON, Rotor OFF with glass wool in port to vacuum (this is to prevent dirt in the tubing from blowing out into Speed Vac when pressure-equilizing later). About 5-10 min until liquid is gone.
- Resuspend in 55 ul of 150 mM KPO4.
Back to Lam
